MedTech
In a radically changing environment, we are making connections across science and technology to combine our own expertise in surgery, orthopaedics and interventional solutions with the big ideas of others to design and deliver physician and patient-centric products and solutions. As pioneers in medical devices, we continually focus on elevating the standard of care – working to expand patient access, improve outcomes, reduce health system costs and drive value. We create smart, people-centered healthcare to help the patients we serve recover faster, live longer and more vibrantly.
Orthopaedics
The Global Orthopaedics business of DePuy Synthes is composed of Joint Reconstruction, Trauma, Spine, Sports Medicine, Power Tools, Craniomaxillofacial (CMF) and Biomaterials. Collectively, these businesses are focused on helping patients along the care continuum – from early intervention to surgical replacement, with the goal of helping people return to living active and fulfilling lives.
Visit the DePuy Synthes Website
 Joint Reconstruction
Joint ReconstructionA global leader in joint replacement, we offer a comprehensive portfolio of hip, knee and shoulder replacements, operating room products and bone cement and accessories.
 Trauma
TraumaWe are the global leader in medical devices used to treat orthopaedic trauma. The company's fixation products, including screws, plates, nails and other implants, are used to treat fractures and deformities related to the extremities and pelvis.
 Spine
SpineOffering one of the most comprehensive portfolios of spinal care solutions in the world, treatment options are available in key areas including thoracolumbar, cervical, aging spine, interbody fusion and arthroplasty.
 Sports Medicine
Sports MedicineDePuy Synthes Mitek is a global leader in orthopaedic sports medicine devices and products used in the treatment of traumatic and degenerative soft tissue pathologies and joint injuries related to sports and physical activity.
 Power Tools
Power ToolsWe offer a comprehensive range of air, electric and battery-driven systems and associated dissections tools in three main areas: Large Bone, Small Bone and High Speed.
 Craniomaxillofacial (CMF)
Craniomaxillofacial (CMF)We offer a comprehensive portfolio of products to treat conditions affecting the face, head and thorax. Our focus is on advancing breakthrough therapies for patients, particularly in the field of personalized patient solutions.
 Biomaterials
BiomaterialsProviding a wide range of innovative products that complement the use of traditional metal implants in trauma, spine and cranio-maxillofacial surgical procedures, our biomaterials assist orthopaedic surgeons around the world with their mission to repair, protect and strengthen the structural core of the human body.
Surgery
In hospitals around the world, surgeons operate with confidence using trusted surgical systems and instruments designed to provide the safest and most effective treatment for a range of medical conditions.
 Ethicon
EthiconFrom creating the first sutures, to revolutionizing surgery with minimally invasive procedures, Ethicon has made significant contributions to surgery for more than 100 years with its broad portfolio that includes: Biosurgery, Endomechanical, Wound Closure, Energy and Robotics.
Visit the Ethicon Website Breast Aesthetics
Breast AestheticsMentor develops, manufactures and markets innovative, science-based products for surgical and non-surgical medical procedures that allow patients to improve their quality of life. Mentor Worldwide LLC is a leading supplier of medical products for the global aesthetic market. The company is focused on two strategic areas—breast and body aesthetics—and is the only manufacturer whose breast implants are made in the U.S.
Visit the Mentor Website
Interventional Solutions
Our medical devices portfolio encompasses a range of innovative business segments that support professionals with tools for treating heart rhythm disorders and neurovascular care.
 Arrhythmias
ArrhythmiasTo treat patients with irregular heartbeats, hospitals and teaching institutions rely on advanced diagnostic, therapeutic and mapping tools from Biosense Webster, Inc. Biosense Webster is the global leader in the science of diagnosing and treating heart rhythm disorders. The company partners with clinicians to develop innovative technologies that improve the quality of care for arrhythmia patients worldwide.
Visit the Biosense Webster Website Neurovascular and Stroke
Neurovascular and StrokeCerenovus is a global leader in neurovascular care. Our commitment to changing the trajectory of stroke is inspired by our long heritage and dedication to helping physicians protect people from a lifetime of hardship. Cerenovus offers a broad portfolio of devices used in the endovascular treatment of hemorrhagic and ischemic stroke.
Visit the Cerenovus Website
Vision
At Johnson & Johnson Vision, we have a big aspiration: help protect the most precious human sense – eyesight.
We bring together cutting-edge insights, science, technology and people to encourage professionals and patients to proactively preserve and enhance sight for life. And in communities with the greatest need, we collaborate to expand access to quality eye care.
Dual-headquartered in Jacksonville, Florida, and Santa Ana, California, we have more than 10,000 employees worldwide. Our eye health products are available in 103 countries.
Visit the Johnson & Johnson Vision Website
-
As the world’s largest, most broadly based healthcare company, we know that human health is inextricably linked to the health of the planet—healthy people need a healthy planet. Our Credo, written in 1943, and our aspiration of advancing health for everyone, everywhere, are at the heart of our commitment to environmental stewardship.
-
How Value-based health care benefits patients and opens up new prospects for the health care system
-
Value Based Health Care - Johnson & Johnson Medical is already putting this concept into practice in Switzerland and brings added value through innovative product solutions: 4S Intelligent Trauma Care stands for more efficient use of resources and higher patient satisfaction.
Medical Devices: 135 Years of Innovation

Johnson & Johnson Founded
Three brothers, Robert Wood Johnson, James Wood Johnson and Edward Mead Johnson, founded Johnson & Johnson in New Brunswick, New Jersey, U.S.

First Mass Production of Sterile Sutures
Johnson & Johnson’s mass production of sterile sutures—along with sterile surgical dressings, cotton and gauze—ushers in the widespread practice of modern antiseptic surgery, dramatically raising survival rates.

DePuy Manufacturing Company Is Founded
Revra DePuy founded the DePuy manufacturing company, the world’s first orthopaedic company.

Ortho Research Laboratories, Inc. Established
The Company expands to Argentina and Brazil. Ortho Research Laboratories, Inc. is established in Linden, New Jersey to make women’s health products.
Ethicon, Inc. Is Formed
Ethicon Suture Laboratories, formed in 1949 from the company’s heritage suture business, formed and became Ethicon, Inc.

Frontier Contact Lens Company is Founded
Johnson & Johnson Vision Care, Inc. begins as Frontier Contact Lens Company in Buffalo, New York before eventually relocating to Jacksonville, Florida a few years later.

Minimizing Blood Loss During Surgery
SURGICEL® Absorbable Hemostat sets a new standard for managing surgical bleeding—by helping to minimize blood loss.

Partnering to Improve Patient Outcomes
The DePuy Synthes Companies of Johnson & Johnson begin their partnership with the AO Foundation to deliver world-class professional education and develop new innovations that improve patient outcomes and increase efficiency of care.
Ethicon’s First Synthetic Suture
Ethicon introduces its first synthetic sterile suture, PROLENE® Polypropylene Suture, which remains the industry standard for cardiac bypass surgery for its strength, secure knotting and biocompatibility.

First Disposable Skin Stapler Introduced
Ethicon’s game-changing skin stapler introduces disposable instruments to the operating room. Smaller and lighter than reusables, the PROXIMATE® disposable skin stapler offers improved visibility and easier use.

New Coated Sutures Become Industry Standard
Adding a coating to the absorbable, polymer-based VICRYL® (polyglactin 910) Suture introduced in 1974, Ethicon shifted the global suture market. Innovations like VICRYL® Suture have helped cement Ethicon’s position as the worldwide leader in sutures.

First Mobile Bearing Knee System Introduced in the U.S.
DePuy introduces the LCS® Mobile Bearing Knee System. At the time, it is the only mobile bearing knee system approved for use in the United States.

Acquisition of Frontier
Johnson & Johnson acquires Frontier.
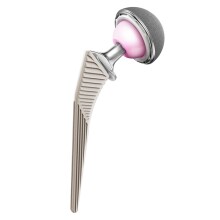
CORAIL Total Hip System Launches
The hip stem is now the world’s most widely used cementless femoral system, which features a bone-conserving surgical technique.

First Disposable Soft Contact Lens
J&J Vision transforms vision correction and the eye care industry by introducing the world’s first disposable soft contact lenses under the name ACUVUE® Brand Contact Lenses.

First Minimally Invasive Gall Bladder Removal
With the first minimally invasive gall bladder removal, Ethicon maximized the potential of laparoscopic procedures. The company quickly began working with leading surgeons to identify minimally invasive surgical solutions.

Giving Surgeons Confidence to Conduct Minimally Invasive Surgeries
The LIGACLIP® ERCA fully automatic clip appliers feature groundbreaking holding power, giving surgeons the confidence to adopt minimally invasive approaches.

Johnson & Johnson Vision Goes Global
J&J Vision offers eye health products and services to eye care professionals and patients in more places around the world by opening an operating group in Tokyo, Japan before further expanding to five continents within the year.
Training the Next Generation of Surgeons
Ethicon opens its first Endo-Surgery Institute in Cincinnati, Ohio, to help surgeons master minimally invasive surgery techniques.

ACUVUE® Brand Contact Lenses Climbs to #1
J&J Vision ACUVUE® Brand Contact Lenses become the #1 most prescribed contact lens brand in the world, helping people see better, connect better, live better.

Providing Superior Comfort and Convenience to Contact Lens Wearers Worldwide
J&J Vision revolutionizes its product portfolio by launching 1-DAY ACUVUE® Brand Contact Lenses, the world’s first daily disposable contact lens.

Driving the Use of Telesurgery
Ethicon hosts the first live telesurgery at the American College of Surgeons’ annual meeting, introducing clinicians to the possibilities of distance learning. Doctors witnessed the real-time broadcast from an off-site operating room.
Elevating Eye Health With Introduction of UV Protection across ACUVUE® Brand Contact Lenses
UV Protection is adopted across the J&J Vision ACUVUE® Brand Contact Lenses portfolio, demonstrating the company’s longstanding commitment to continually raising standards of eye care.

Advancing Cutting & Coagulating
Ethicon introduces the first 5mm HARMONIC® shears, enabling, for the first time, surgeons to dissect, cut and coagulate with one ultrasonic energy device, while also reducing thermal damage and preserving tissue function.

The World’s First Topical Skin Adhesive Introduced
DERMABOND® Topical Skin Adhesive is introduced in the U.S., paving the way for replacing sutures with a new generation of skin closure solutions.

Elevating Eye Health With Introduction of UV Protection across ACUVUE® Brand Contact Lenses
J&J Vision introduces ACUVUE® Brand Contact Lenses Bifocal, providing near vision correction to those with presbyopia.
Advancing Contact Lens Comfort
J&J Vision ACUVUE Brand Contact Lenses introduces their INVISIBLE EDGE™ Design to help the eyelid glide seamlessly across the lens, helping to enhance comfort. The design is implemented across the ACUVUE® Brand Contact Lenses product portfolio.

Fighting Obesity
Entering the new millennium, Ethicon committed to entering a multi-front assault against obesity. Our investment has created groundbreaking research in metabolic science, advanced bariatric training and standards.

Replacing Natural Hip Sockets
The PINNACLE® Acetabular Cup System replaces the natural hip socket (acetabulum) and has since been provided for more than two million patients.

The First Coated Antibacterial Suture Introduced
The first commercially available antibacterial suture, Coated VICRYL® Plus Antibacterial (polyglactin 910) Suture broke new ground in reducing surgical-site infections by preventing bacterial colonization of the suture.

Supporting the Professional Growth of Eye Care Professionals
The first VISION CARE INSTITUTE, LLC is established to provide eye care professionals and aspiring students with education, training and research opportunities. Today there are 15 Institutes across the global network.

DePuy Introduces Injection Treatment for Osteoarthritic Pain
DePuy begins selling ORTHOVISC© High Molecular Weight Hyaluronan, a multi-injection hyaluronan treatment used to treat knee pain due to osteoarthritis. ORTHOVISC© is a registered trademark of and manufactured by Anika Therapeutics.

Helping Maintain a Stable Tear Film for Contact Lens Comfort
J&J Vision ACUVUE OASYS® with HYDRACLEAR® PLUS Technology is launched, introducing a new lens wetting technology with increased moisture throughout. ACUVUE OASYS® will later introduce new options for people with astigmatism and presbyopia.

FDA Approves First-Ever Silicone Gel Implants
FDA approves Mentor’s MemoryGel™ Breast Implants in the U.S., representing the first approval of silicone gel implants by the FDA.

J&J Vision Unlocks and Unleashes Proprietary Technology for Exceptional Contact Lens Comfort
1-Day ACUVUE® MOIST Contact Lenses with LACREON® Technology are introduced, locking in the lenses’ wetting agent to provide wearers with a long-lasting cushion of moisture for exceptional comfort.

ACUVUE® Receives the WCO Global Seal of Acceptance
J&J Vision ACUVUE® Brand Contact Lenses become the first to receive the World Council of Optometry’s (WCO) Global Seal of Acceptance for UV Absorbing Contact Lenses.

Providing Astigmatism Patients a J&J Vision ACUVUE Brand Contact Lens Option
J&J Vision ACUVUE OASYS® for Astigmatism launched using ACCELARATED STABILIZATION DESIGN™ (BLINK STABILIZED™ Design in the U.S.) to provide vision correction for those with astigmatism.

Mentor Provides Advances in Breast Tissue
Mentor launches the CONTOUR PROFILE® Tissue Expander with suturing tabs (CPX3), the first of its kind.

J&J Vision Introduces World to First Silicone Hydrogel Daily Disposable Contact Lens
J&J Vision 1-DAY ACUVUE® TruEye® with HYDRACLEAR® Technology launches in the United Kingdom, introducing the world to the first silicone hydrogel daily disposable contact lens. This technology enhances oxygen flow to the eye, providing superior comfort comparable to wearing no lenses.
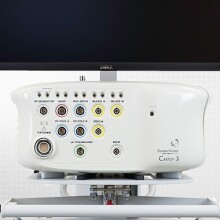
Biosense Webster Introduces Most Advanced Mapping System
Biosense Webster introduces CARTO® 3, the most advanced and most used 3D Cardiac Electro-anatomical Mapping System.

Biosense Webster Provides Significant Advancements for the Clinical Community
Biosense Webster launches THERMOCOOL SMARTTOUCH® SF Catheter.

Acclarent Launches Single-Handed Balloon Sinuplasty Device
Acclarent launches RELIEVA®Spin, a single-handed Balloon Sinuplasty device for in-office and operating room sinus surgery.

Johnson & Johnson Acquires Synthes, Inc.
In a $21.3 billion transaction, the subsequent integration of the Synthes and DePuy businesses creates the world’s most comprehensive orthopedic and neurological company.

Knees to Get Patients Back to Enjoying Life
DePuy Synthes Companies launches the ATTUNE® Knee System, designed to help patients get back to what they enjoy sooner by optimizing how a patient’s knee replacement feels and moves.

Harmonic ACE+7 Shears With Advanced Hemostasis
The first purely ultrasonic device with a 7mm sealing indication, the breakthrough device combined precision and reliable sealing for large vessels. This dual-duty device allows surgeons to remain focused on the procedure.

Ten Years of Clinical Data Leads to Extended Gel Breast Portfolio
Mentor’s extended gel breast portfolio with U.S. launch of shaped implants in U.S. supported by 10 years of clinical data.

Helping Surgeons Stop Problematic Bleeding
EVARREST® Fibrin Sealant Patch is engineered to rapidly and reliably help surgeons stop bleeding in problematic situations when conventional methods are ineffective.
Important Safety Information

Providing New Options for Physicians
The MENTOR® ARTOURA™ Breast Tissue Expander is introduced and is the first expander with dynamic control technology for predictable expansion.
Johnson & Johnson/Ethicon Definitive Agreement With Google to Advance Surgical Robotics
Strategic collaboration designed to develop new robotic technologies that offer surgeons and health care systems greater accuracy, cost efficiency and better outcomes for patients.

Ethicon Acquires NeuWave Medical, Inc.
Ethicon acquired NeuWave Medical, Inc., a company that manufactures and markets soft tissue microwave ablation systems. This gave Ethicon a strong, minimally invasive entry into ablating soft tissue lesions.

Formation of Verb Surgical in Collaboration With Verily (formerly Google Life Sciences)
The new company was formed by the strategic collaboration between Ethicon and Verily. In partnership with surgeons around the world, Verb Surgical aims to develop the next evolution in robotic surgery.

J&J Vision Helps Make the Feeling of Tired Eyes a Thing of the Past
J&J Vision launches ACUVUE OASYS® 1-DAY with HydraLuxe™ Technology, new technology for improved comfort and performance in challenging environments. The lens has tear-like properties that work with your natural tear film, providing all-day comfort and performance.

DePuy Synthes Launches TFN-ADVANCED® Nail System
The TFN-ADVANCED® Proximal Femoral Nail System advances hip fracture treatment and solves a wide range of unmet needs for surgeons, OR staff and administrators treating people with these debilitating fractures.

J&J Vision ACUVUE® Brand Contact Lenses Introduces Monthly Contact Lenses with HydraMax™
J&J Vision ACUVUE® VITA Monthly Contact Lenses with HydraMax™ Technology are introduced to help maximize and maintain lens hydration for reliable, month-long comfort.

Acclarent Safely and Effectively Treating ETD
ACCLARENT AERA™ Eustachian Tube Balloon Dilation System is introduced. It’s the first FDA-approved device to safely and effectively treat Eustachian Tube Dysfunction (ETD) vs. medical management.

DePuy Synthes Launches Variable Angle Locking Hand System
The Variable Angle Locking Hand System provides an extensive range of basic and specialty plates for fracture repair and deformity correction designed to reduce potential soft tissue irritation with the stability of locked plating.

Johnson & Johnson Acquires Pulsar Vascular
Johnson & Johnson Medical Devices acquires Pulsar Vascular, a breakthrough platform technology for the neurovascular treatment of complex aneurysms.

Biosense Webster Launches THERMOCOOL SMARTTOUCH® SF Catheter
Biosense Webster announces the U.S. approval of the THERMOCOOL SMARTTOUCH® SF Catheter. The catheter is the only approved device pairing contact force technology with a porous tip designed to optimize efficiency.

Johnson & Johnson Acquires Abbott Medical Optics
J&J acquires Abbott Medical Optics, gaining global leadership in ophthalmic surgery, including the #1 position in LASIK refractive surgery. J&J Vision able to offer broad range of eye health solutions to help eye care professionals and their patients through every stage of the lifetime eye health journey.

DePuy Synthes Launches the VIPER PRIME® System in Spine
The VIPER PRIME® System for minimally invasive spine surgery combines multiple instruments into one tool and enables surgeons to target pedicles and insert screws in one single instrument pass, streamlining the procedure.

Combining Energy and Electrosurgery in the OR
Ethicon’s acquisition of Megadyne Medical Products, Inc. brings together the intelligence of its advanced energy devices and Megadyne’s innovative portfolio of electrosurgical tools.

A Revolutionary Treatment for Reflux Disease
Ethicon’s acquisition of Torax allowed for investments in areas of unmet medical needs such as esophageal health. With its LINX® Reflux Management System, Ethicon is now able to provide surgical treatment for Gastroesophageal reflux disease. View safety information

Johnson & Johnson Acquires Neuravi
Johnson & Johnson Medical Devices acquires Neuravi Limited, a company dedicated to advancing neurovascular therapies and improving clinical outcomes for acute ischemic stroke patients.

Johnson & Johnson Launches CERENOVUS to Advance Stroke Treatment
Johnson & Johnson Medical Devices Companies introduced CERENOVUS, its new neurovascular business.

J&J Vision Leading LASIK Technology iDESIGN Refractive Studio Launches
J&J Vision launches the iDESIGN Refractive Studio, the first and only topo-integrated, wavefront-guided technology for LASIK.

Johnson & Johnson Medical Devices Acquires Orthotaxy
Johnson & Johnson Medical Devices Companies acquired Orthotaxy, developer of a differentiated robotic surgery platform for orthopaedic procedures.

DePuy Synthes Launches ACTIS® Total Hip System
The ACTIS® Total Hip System is designed to increase joint stability immediately after hip replacement surgery and optimized for the Anterior Approach, which helps patients get back on their feet more quickly following surgery.

DePuy Synthes Introduces KINCISE™ Surgical Automated System
The KINCISE™ Surgical Automated System is a battery-powered device that automates key steps in total hip replacement surgery, leading to more consistent clinical outcomes, helping to reduce surgeon fatigue and the potential for work-related injuries.

DePuy Synthes Launches DYNACORD™ Suture
This high-strength suture is uniquely designed to respond to changes in tension.

Changing the Game for Continuous Oozing Bleeding With SURGICEL® Powder
Ethicon improves the standard of care with a powdered version of its legacy adjunctive hemostat, SURGICEL® Original, to address a very common type of bleeding during surgery.

CERENOVUS Receives FDA Clearance for EMBOTRAP II
CERENOVUS receives 510(k) clearance from the FDA for EMBOTRAP II Revascularization Device, a next generation stent retriever used to capture and remove life-threatening blood clots from the brain following an ischemic stroke.

Acclarent Launches TruDi
Acclarent launches TruDi, a real time, three-dimensional (3D) navigation system for ENT procedures performed in the operating room and surgeon office.

J&J Vision ACUVUE® OASYS Launches The Contact Lens That Knows Light™
Johnson & Johnson launches ACUVUE® OASYS with Transitions™ Light Intelligent Technology™, the first-of-its-kind contact lens that seamlessly adapts to changing light, both revolutionizing the way contact lenses are worn and earning one of TIME’s Best Inventions of 2018.

Johnson & Johnson Vision Launches Next-Generation Monofocal IOL for Patients With Cataracts in Europe
Breakthrough innovation J&J Vision TECNIS® Eyhance IOL first lens in the monofocal IOL category in Europe to deliver improved intermediate vision and 20/20 distance vision; offers same, well-established low incidence of halo, glare, or starburst as TECNIS® 1-piece IOLs.

Johnson & Johnson Acquires Auris Health, Inc.
Auris Health’s robotic platform technology will enable Johnson & Johnson to deliver on the promise of digital surgery and drive better outcomes for patients.

Biosense Webster Launches CARTONET
Johnson & Johnson Companies launches CARTONET as part of the company’s mission to help revolutionize the way health systems leverage data with a goal of improving patient outcomes and operational efficiency in catheter ablation procedures.

Advanced Stapling for Circular Anastomosis With ECHELON CIRCULAR Powered Stapler
The product offers advanced stapling technologies that target circular anastomosis, a critical step in surgery.

DePuy Synthes Advances Treatment Options for Patients with Complex Cervical Spine Disorders
DePuy Synthes launched the SYMPHONY™ Occipito-Cervico-Thoracic (OCT) System, expanding its offering for the surgical treatment of conditions in the neck and upper back.
1886

1887

1895

1937

1949
1959

1960

1960

1969
1978

1979

1980
1981

1985

1987

1988

1991

1991

1992
1993

1993

1995

1997
1998

1998

1998

1999
2000

2001

2003

2004

2005

2005

2006

2006

2007

2007

2008

2008

2009

2011

2012

2012

2013

2014

2014

2014

2015

2015
2015

2015

2015

2015

2016

2016

2016

2016

2016

2017

2017

2017

2017

2017

2017

2018

2018

2018

2018

2018

2018

2018

2018

2019

2019

2019

2019

2019

2019





